Microbiology 102: Flow Charts, Tables and Dichotomous Keys
|
I. Preparation of "Flow Charts" – relevant to poster presentations and lab preparation in general.Note: Flow Charts and other details regarding "Materials and Methods" are not required in reports for Microbiology 102, as a simple referral to our lab manual can be done as per our report guidelines. 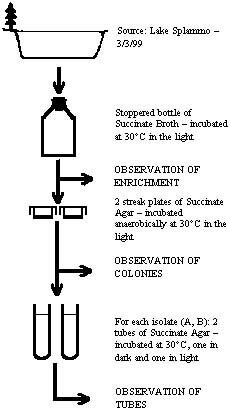
As a good table can collect and organize the observations and results obtained for various known and unknown organisms studied in the lab, a good flow chart can present a concise overview of the relationships of the various activities that were done in the experiment. Part of one's lab preparation (i.e., coming to lab prepared!) should be checking the schedule to see what is coming up and looking closely at the various activities to be performed. Nothing beats some good flow charts to help you plan your work efficiently, especially when there are several different experiments happening in the same lab period. When making a flow chart, one must avoid connecting all of the activities performed in linear fashion. Rather than copying the directions in the manual verbatim and then connecting sentences or paragraphs with arrows, you can improve upon those directions – making sure you are retaining important inoculation and incubation directions and also refraining from excess wordiness. If one makes wet mounts of an enrichment and then streaks plates from the enrichment, there should not be an arrow drawn between the two activities as if to show the plates were streaked from wet mounts! A flow chart can be made as general or specific as one sees fit. The example illustrated on the right gives a general overview of the procedure for our Experiment 11B wherein purple non-sulfur photosynthetic bacteria are isolated by enrichment and plating. This is a continuous flow chart, not divided into the several "periods" as indicated in the lab manual for this experiment. This kind of general flow chart is appropriate for posters, making for easy connection by the poster-viewer between procedures and results at various stages of the experiment, and all essential parts of the procedure are represented. Note the orientation toward materials being connected with the arrows. The methods are indicated as concisely as possible; the author of this flow chart does not label specifically the inoculations if they appear understood. Set off laterally are indications where observations and/or tests are made (the results being summarized in the "Results" section of the poster). The following general outline for Experiment 11C (isolation of Bacillus) may be considered a flow chart and can be made more specific by indicating incubation conditions (time and temperature) and where observations were made (summarized elsewhere in appropriate tables). The use of indentations can show relationships between the various things set up in the procedure.
II. Preparation of Tables – relevant to lab reports, poster presentations and the tabulation of results in general.When it comes to indicating results in a report or poster, the observations of enrichments (which are always mixed cultures, no matter how "selective" the enrichment) should always be kept separate from observations and reactions of the individual isolates. For example, in Exp. 11B on the purple non-sulfur photosynthetic bacteria, one could describe one's observations of the enrichment as follows: "Red, cloudy growth observed throughout the bottle. Wet mounts showed motile and non-motile rods and oval cells, and motile spirilla." Then, once isolates are obtained (that is, once individual colonies are seen and considered for further study), the recording of information concerning them (and their subcultures) should be put in a table – an example of which is shown below. Note that macroscopic and microscopic observations on the colonies are included as well as the results of further tests made on cultures inoculated from the same colonies. Tentative genus identifications can be included based on cellular morphology. |
| isolate designation |
colony characteristics |
cellular morphology |
growth in Succinate Agar tubes | tentative identification |
||
| in light | no light | conclusion | ||||
| A | tiny, red, hard | oval cells connected by filaments | no aerobic growth; anaerobic growth with red pigmentation | no growth | strict phototroph |
Rhodomicrobium |
| B | large, orange, mucoid | curved, knobby rods | white aerobic growth; red anaerobic growth | white aerobic growth; no anaerobic growth | facultative phototroph |
Rhodopseudomonas |
III. Preparation of Dichotomous Keys – relevant to differention and identification of bacteria.A dichotomous key shows how various biological entities can be differentiated from each other by indicating their opposing reactions in carefully-chosen tests and observations. In a previous biology course, you were probably exposed to such keys written out in paragraph form such as in the following example, adapted from the very old and outdated text Freshwater Algae of the United States by Gilbert M. Smith (McGraw-Hill, 1950):
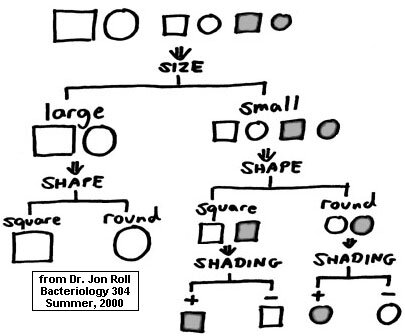
With the first pair of observations (numbered "1"), one genus (Distigma) is immediately differentiated from the others by being the only one with two flagella per cell. Those genera with one flagellum need further differentiation which is accomplished by succeeding pairs of observations (numbered "2" and "3"); sooner or later, all of the genera are differentiated from each other. Dichotomous keys can also apply to non-biological entities as shown in the following example where six objects are fully differentiated based on some obvious physical characteristics. In this key, the inverted tree form is followed, and each individual object is at the very end of its own "branch."
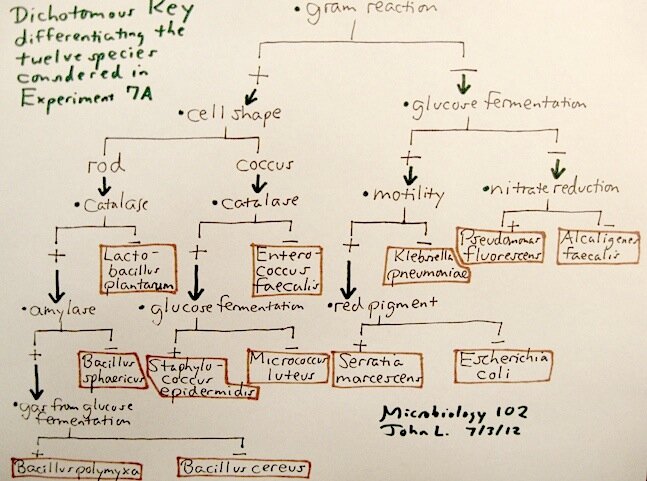
In bacteriology, such keys are occasionally seen, but it cannot be emphasized enough that differentiation of genera – such as in the multi-genera Family Enterobacteriaceae – is not always so clear-cut, and a table showing the reactions (sometimes "variable") of the many tests necessary to differentiate the organisms is often preferred over a dichotomous key. Go to such texts as Bergey's Manual or the Manual of Clinical Microbiology to see many examples of such tables. Back in our course, we can illustrate the usefulness of a dichotomous key to differentiate the twelve known cultures in Experiment 7A. One could construct such a key for our unknown identification experiments such that relevant testing and ultimate identification of the possible unknown organisms are facilitated. The following shows what we may come up with for Exp. 7A:
There is no rule that says any particular test or observation has to be found at the same level across the width of the key, but the most "primary" tests should be used first (such as gram reaction, cellular morphology, catalase reaction, glucose fermentation) before other tests are utilized (lactose fermentation, amylase, etc.). Dichotomous keys must not be confused with flow charts (above). One can run any number of tests at any given time and obtain results for more than one level of the key. |
||||||||||||||